ReceptIVFity
ReceptIVFity
ReceptIVFity is a predictive test for assessing the current suitability of an individual woman’s embryo implantation acceptation, prior to starting an IVF treatment – with or without ICSI – based on vaginal microbiome measurements.
Scientific research has demonstrated that the composition of bacteria in the vagina (the vaginal microbiome) plays an important role in the chances of achieving a successful pregnancy using fertility treatments such as IVF or ICSI.
Clinical trial
During the ReceptIVFity-I trial (2014-2016), the results of earlier single-center clinical studies have been optimized in a multicenter setting. For this study 300 patients were included originating from 8 Dutch IVF clinics. A secondary purpose of the ReceptIVFity-I trial design was to technically compare different high-throughput platform technologies in order to choose the one most suitable for the ReceptIVFity test. These technologies enable personalized, quick, robust and specific predictions. The outcome of the ReceptIVFity-I trial clearly demonstrates the capacities of the urogenital microbiome composition as a predictor for pregnancy outcome after IVF/ICSI.
Before the start the IVF or ICSI treatment, the patient will collect some mucus from the vagina using a swab from the test pack. After collecting the sample, she will place the cotton swab in the tube with liquid that has been provided and give this tube to the physician. The tube with the sample will then be sent to the laboratory. In the laboratory, the bacterial DNA collected from this sample will be analyzed – quantitatively and qualitatively – and will determine the woman’s individual microbiome profile. The physician can check the result from the lab within 7 working days here and will discuss the results with the patient.

The result
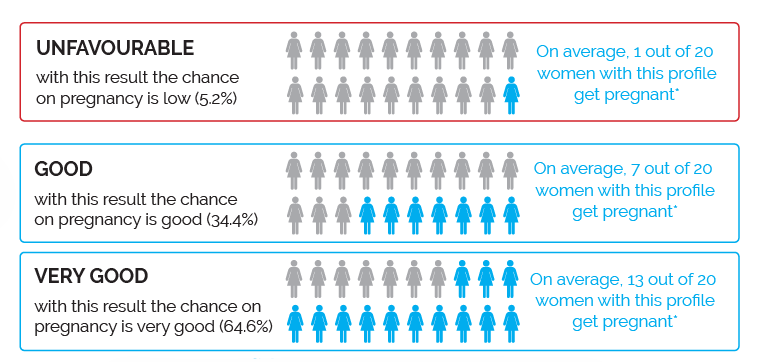
The ReceptIVFity test determines the profile of the bacterial balance in the vagina and can result in an unfavourable, good or very good score.
- One out of 20 women gets an UNFAVOURABLE score; this means the chance of becoming pregnant after an IVF or ICSI treatment is low (5.2%)*.
- On average, 7 out of 20 women get a GOOD score, in which case the chance of pregnancy is 34.4%*.
- On average, 13 out of 20 women get a VERY GOOD score, in which case the chance of pregnancy is high (64.6%)*.
*% of confirmed pregnancies by ultrasound after first embryo transfer following sampling
Decision making process
When a patient has a high ReceptIVFity score, this does not mean that she will definitely become pregnant, but the chances are good. On average, the first IVF or ICSI treatment has just over 30% chance of resulting in a pregnancy and a high score profile increases this chance to 50%.
If the test results in a low score, the patient can consult her doctor about the options. It is important to realise that the proven reliability of the test is for a limited period of maximum two months following the test. The bacterial profile can change over time. After consulting the doctor, and based on the patient’s personal circumstances, she decides whether or not to start IVF or ICSI treatment at that time.
Being able to assess a low chance situation, ReceptIVFity enables doctors and patients to optimize the timing of IVF-treatments. The test also helps to reduce patients’ uncertainty and stress by disclosing more knowledge upfront.
Further developments
For the development of the CE-certified version of ReceptIVFity, top scientists have been involved to help select the most optimal urogenital sampling sites and procedures, as well as the most suitable technology for high-throughput analyses. Furthermore the research team has optimized the algorithm-steered prediction and created a personalized analysis report that will accompany each analysis. The ReceptIVFity test is currently available in several fertility clinics. For more information and latest news on the test, please visit ReceptIFVity.com.
The microbiome profiling technique enables us to search specifically for solutions for women with an unfavorable microbiome. At the moment, various studies are being conducted to investigate whether the use of medication or probiotics has a beneficial effect on the microbiome. Want to know more about our Research & Development projects? Please keep reading.
